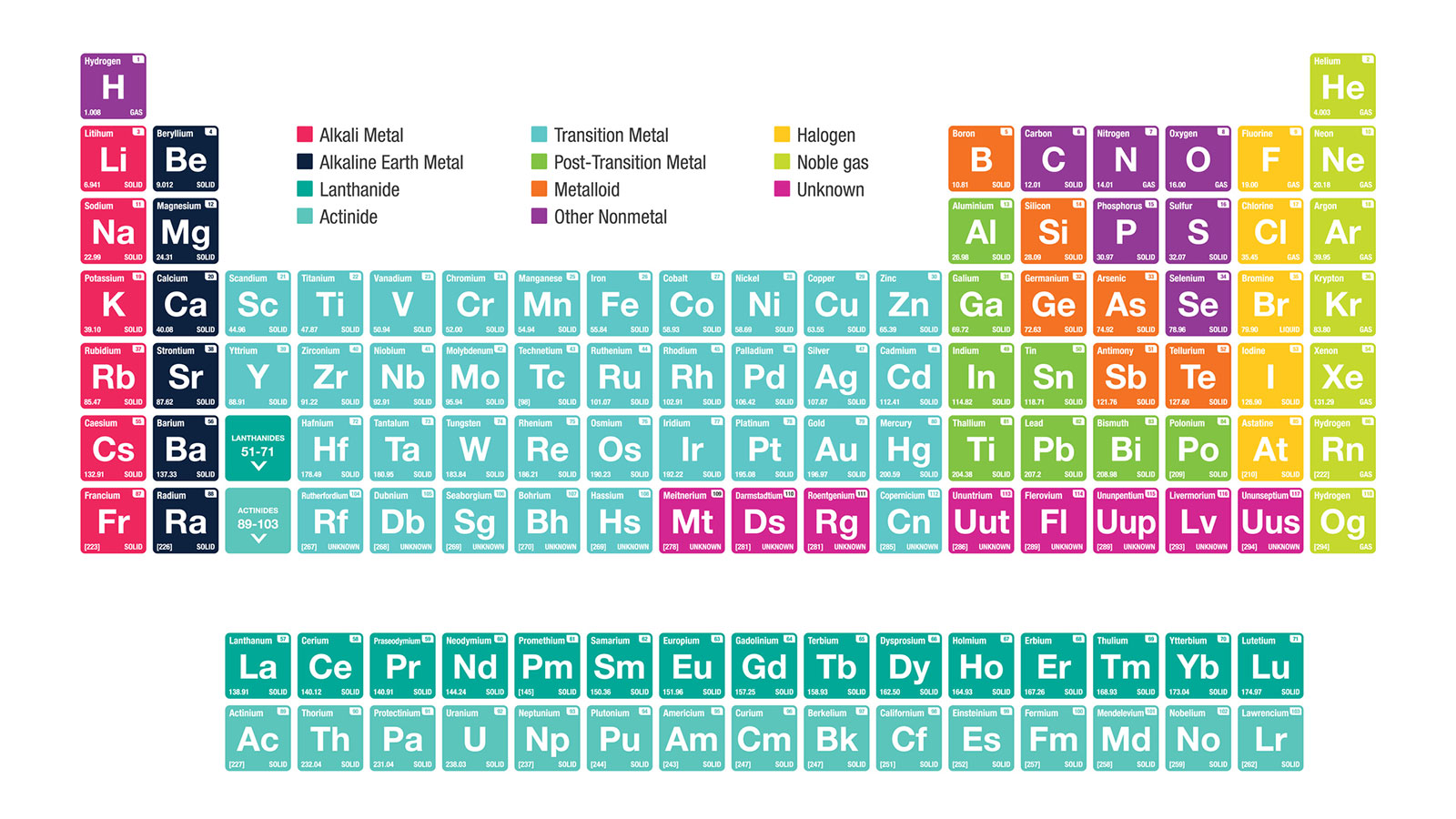
What Properties Do Alkali Metals Have In Common . Every element has a nucleus, made up of. The alkali metals exhibit many of the physical properties. the lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals have the high thermal and electrical conductivity,. chemical properties of alkali metals. alkali metal properties. the alkali metals tend to form +1 cations.
from elchoroukhost.net
The alkali metals have the high thermal and electrical conductivity,. alkali metal properties. The alkali metals exhibit many of the physical properties. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. the lone outer shell electrons leads the alkali metal elements to share several common properties: the alkali metals tend to form +1 cations. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. chemical properties of alkali metals. The group 1 elements are all soft, reactive metals with low melting points. Every element has a nucleus, made up of.
Alkali Metals Periodic Table Properties Elcho Table
What Properties Do Alkali Metals Have In Common chemical properties of alkali metals. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. Every element has a nucleus, made up of. The alkali metals have the high thermal and electrical conductivity,. the lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals exhibit many of the physical properties. the alkali metals tend to form +1 cations. The group 1 elements are all soft, reactive metals with low melting points. alkali metal properties. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. chemical properties of alkali metals.
From www.chemistry4students.com
Chemistry 4 Students Alkali Metals (group 1 elements) What Properties Do Alkali Metals Have In Common The group 1 elements are all soft, reactive metals with low melting points. alkali metal properties. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. The alkali metals exhibit many of the physical properties. The alkali metals have the high thermal and electrical conductivity,. the physical and chemical. What Properties Do Alkali Metals Have In Common.
From studylib.net
alkali metals What Properties Do Alkali Metals Have In Common Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. the alkali metals tend to form +1 cations. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. The alkali metals have the high thermal and. What Properties Do Alkali Metals Have In Common.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation, free What Properties Do Alkali Metals Have In Common The group 1 elements are all soft, reactive metals with low melting points. The alkali metals exhibit many of the physical properties. The alkali metals have the high thermal and electrical conductivity,. alkali metal properties. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which.. What Properties Do Alkali Metals Have In Common.
From thechemistrynotes.com
Comparison of properties of Alkali and Alkaline Earth Metals What Properties Do Alkali Metals Have In Common The alkali metals have the high thermal and electrical conductivity,. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. alkali metal properties. The alkali metals exhibit many of the physical properties. the alkali metals tend to form +1 cations. The group 1 elements. What Properties Do Alkali Metals Have In Common.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions What Properties Do Alkali Metals Have In Common the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. The alkali metals exhibit many of the physical properties. The alkali metals have the high thermal and. What Properties Do Alkali Metals Have In Common.
From www.slideshare.net
Physical and chemical properties of alkali metals What Properties Do Alkali Metals Have In Common the alkali metals tend to form +1 cations. the lone outer shell electrons leads the alkali metal elements to share several common properties: the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. chemical properties of alkali metals. Cation formation is favored by. What Properties Do Alkali Metals Have In Common.
From pediaa.com
Difference Between Alkali Metals and Alkaline Earth Metals Definition What Properties Do Alkali Metals Have In Common Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. chemical properties of alkali metals. The alkali metals have the high thermal and electrical conductivity,. The group 1 elements are all soft, reactive metals with low melting points. Every element has a nucleus, made up of. the alkali metals. What Properties Do Alkali Metals Have In Common.
From overallscience.com
Trends in atomic and physical properties of alkali metals Overall Science What Properties Do Alkali Metals Have In Common the alkali metals tend to form +1 cations. Every element has a nucleus, made up of. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. The alkali metals have the high thermal and electrical conductivity,. The alkali metals exhibit many of the physical properties.. What Properties Do Alkali Metals Have In Common.
From dxopzrtme.blob.core.windows.net
What Do Alkali Metals And Alkaline Earth Metals Have In Common at What Properties Do Alkali Metals Have In Common the alkali metals tend to form +1 cations. the lone outer shell electrons leads the alkali metal elements to share several common properties: The alkali metals have the high thermal and electrical conductivity,. The alkali metals exhibit many of the physical properties. alkali metal properties. chemical properties of alkali metals. the physical and chemical properties. What Properties Do Alkali Metals Have In Common.
From dxozzbgbz.blob.core.windows.net
Chemical Properties Of Alkali Metals Ppt at Crystal Gilbert blog What Properties Do Alkali Metals Have In Common The group 1 elements are all soft, reactive metals with low melting points. The alkali metals have the high thermal and electrical conductivity,. chemical properties of alkali metals. the lone outer shell electrons leads the alkali metal elements to share several common properties: the alkali metals tend to form +1 cations. alkali metal properties. Every element. What Properties Do Alkali Metals Have In Common.
From assign.unaux.com
Periodic properties of alkali metals and halogens. assign What Properties Do Alkali Metals Have In Common Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. The alkali metals have the high thermal and electrical conductivity,. the lone outer shell electrons leads the alkali metal elements to share several common properties: The group 1 elements are all soft, reactive metals with low melting points. the. What Properties Do Alkali Metals Have In Common.
From sciencenotes.org
Alkali Metal Properties What Properties Do Alkali Metals Have In Common alkali metal properties. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. Every element has a nucleus, made up of. chemical properties of alkali metals. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier. What Properties Do Alkali Metals Have In Common.
From period-faqs.com
Where Are The Alkali Metals On The Periodic Table What Properties Do Alkali Metals Have In Common the lone outer shell electrons leads the alkali metal elements to share several common properties: chemical properties of alkali metals. The alkali metals exhibit many of the physical properties. Every element has a nucleus, made up of. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. The alkali. What Properties Do Alkali Metals Have In Common.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 What Properties Do Alkali Metals Have In Common chemical properties of alkali metals. the alkali metals tend to form +1 cations. Every element has a nucleus, made up of. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. The alkali metals exhibit many of the physical properties. alkali metal properties.. What Properties Do Alkali Metals Have In Common.
From xlskoor.blogspot.com
Alkali Metals Chemistry What Properties Do Alkali Metals Have In Common the lone outer shell electrons leads the alkali metal elements to share several common properties: the alkali metals tend to form +1 cations. alkali metal properties. The alkali metals have the high thermal and electrical conductivity,. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. the. What Properties Do Alkali Metals Have In Common.
From dxofbbfye.blob.core.windows.net
What Are The Common Uses Of Alkali Metals at Deborah Summers blog What Properties Do Alkali Metals Have In Common The group 1 elements are all soft, reactive metals with low melting points. Every element has a nucleus, made up of. chemical properties of alkali metals. the physical and chemical properties of the alkali metals can be readily explained by their having an ns 1 valence electron configuration, which. the alkali metals tend to form +1 cations.. What Properties Do Alkali Metals Have In Common.
From www.sliderbase.com
Element Classes Presentation Chemistry What Properties Do Alkali Metals Have In Common the lone outer shell electrons leads the alkali metal elements to share several common properties: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to. The group 1 elements are all soft, reactive metals with low melting points. chemical properties of alkali metals. the alkali metals tend to. What Properties Do Alkali Metals Have In Common.
From elchoroukhost.net
Properties Of Alkali Metals On The Periodic Table Elcho Table What Properties Do Alkali Metals Have In Common alkali metal properties. the lone outer shell electrons leads the alkali metal elements to share several common properties: Every element has a nucleus, made up of. The alkali metals exhibit many of the physical properties. chemical properties of alkali metals. the physical and chemical properties of the alkali metals can be readily explained by their having. What Properties Do Alkali Metals Have In Common.